Research overview
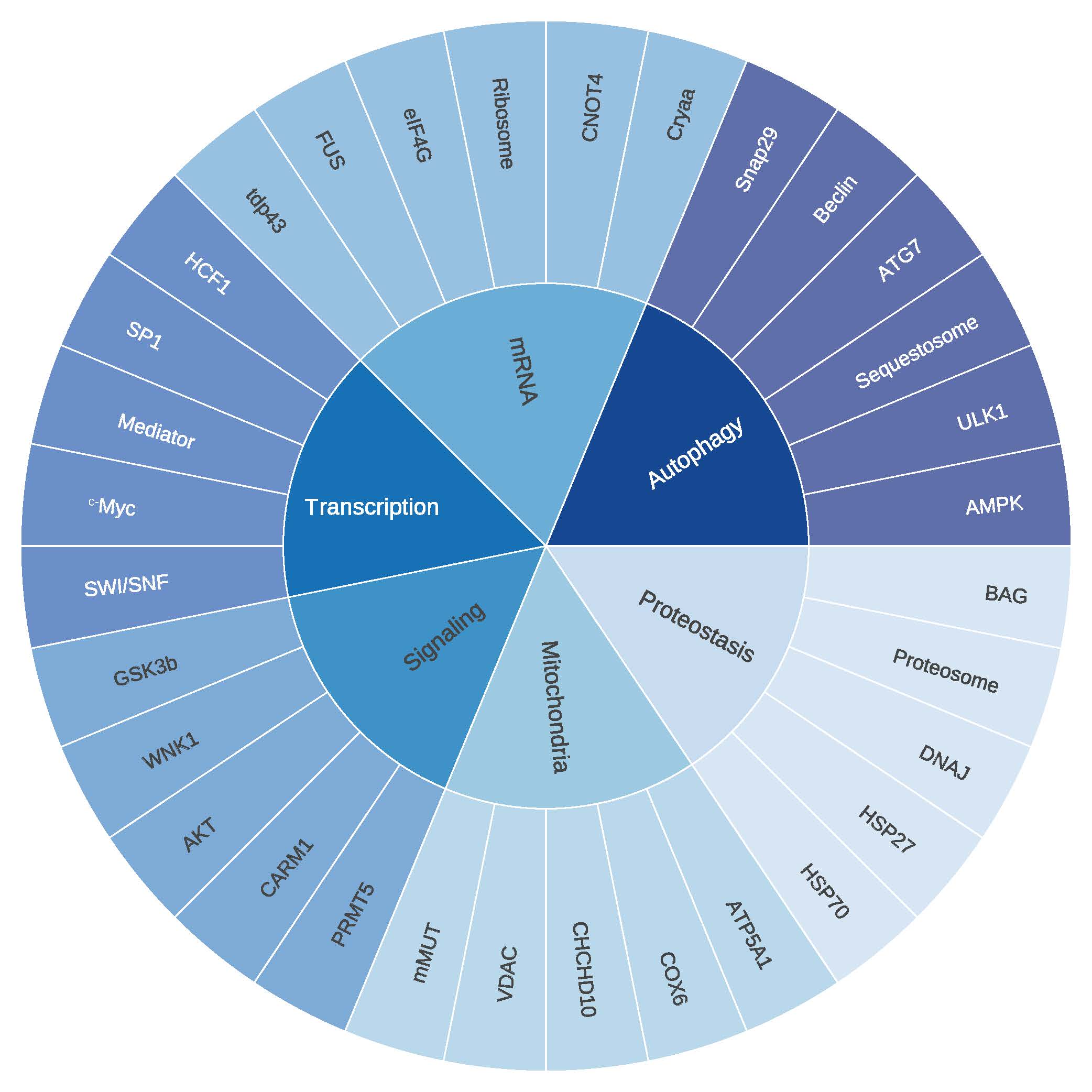
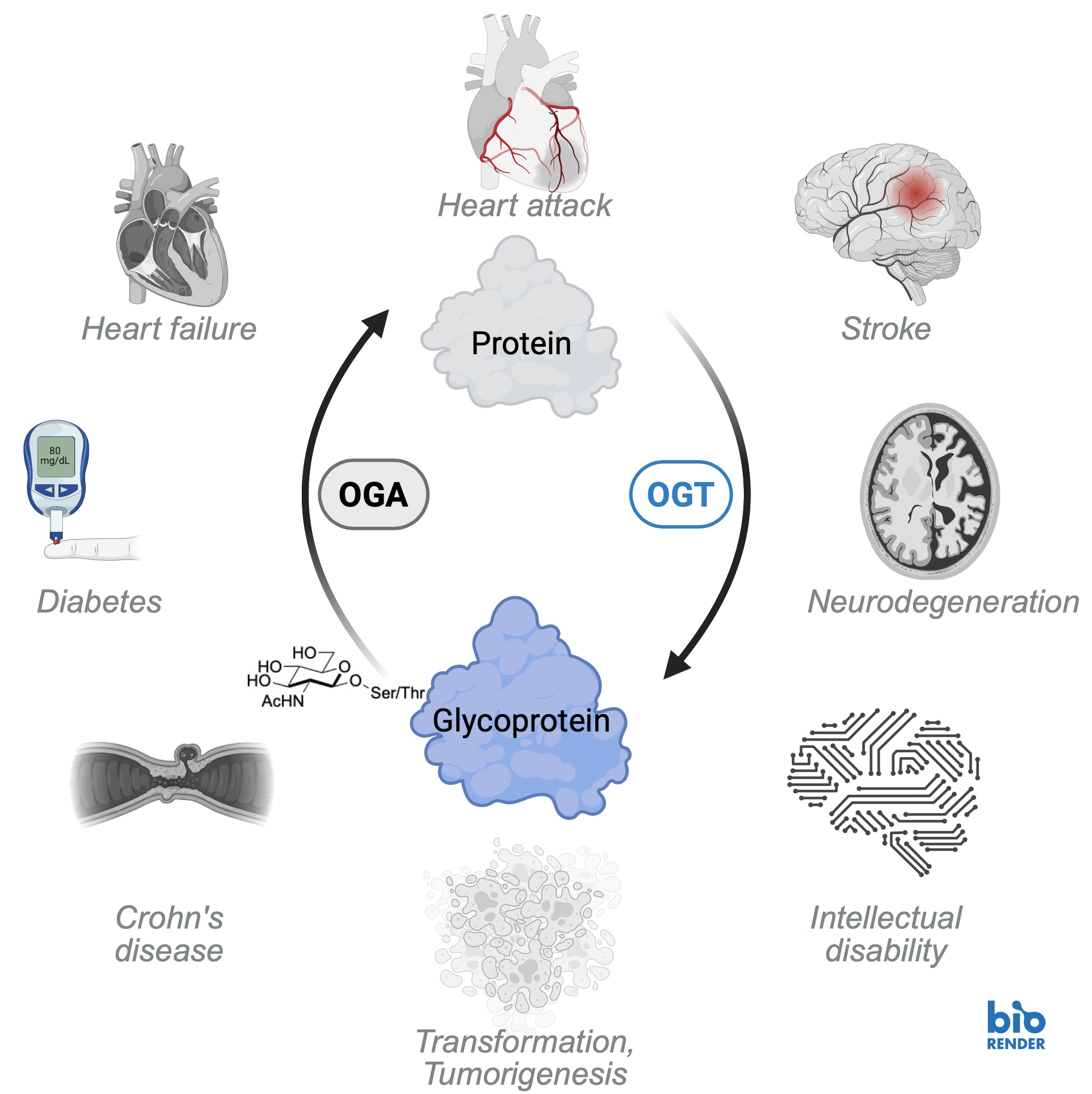
O-GlcNAc is a post-translational modification found on thousands of intracellular proteins, including transcription factors, kinases, and cytoskeletal components. It plays a critical role in regulating diverse cellular processes such as protein folding and stability, localization, activity, post-translational modifications, and protein-protein interactions. Cells coordinate these molecular events across a wide array of proteins in response to environmental and physiological cues, thereby fine-tuning epigenetics, transcription, translation, signal transduction, cell cycle progression, and metabolism. The cellular stress response is no exception – various forms of injury trigger dynamic changes in the O-GlcNAc-subproteome that promote survival. Research in our laboratory broadly focuses on two key questions:
- Which proteins are dynamically modified by O-GlcNAc in response to injury, and how does this modification alter their function to support cell survival?
- How are the enzymes that add and remove O-GlcNAc regulated during cellular stress?
We address these questions using a combination of traditional biochemical and glycobiology techniques, high-throughput technologies, and genetic manipulation.